Our Science
Why SCG3 is better?
Disease-specific:
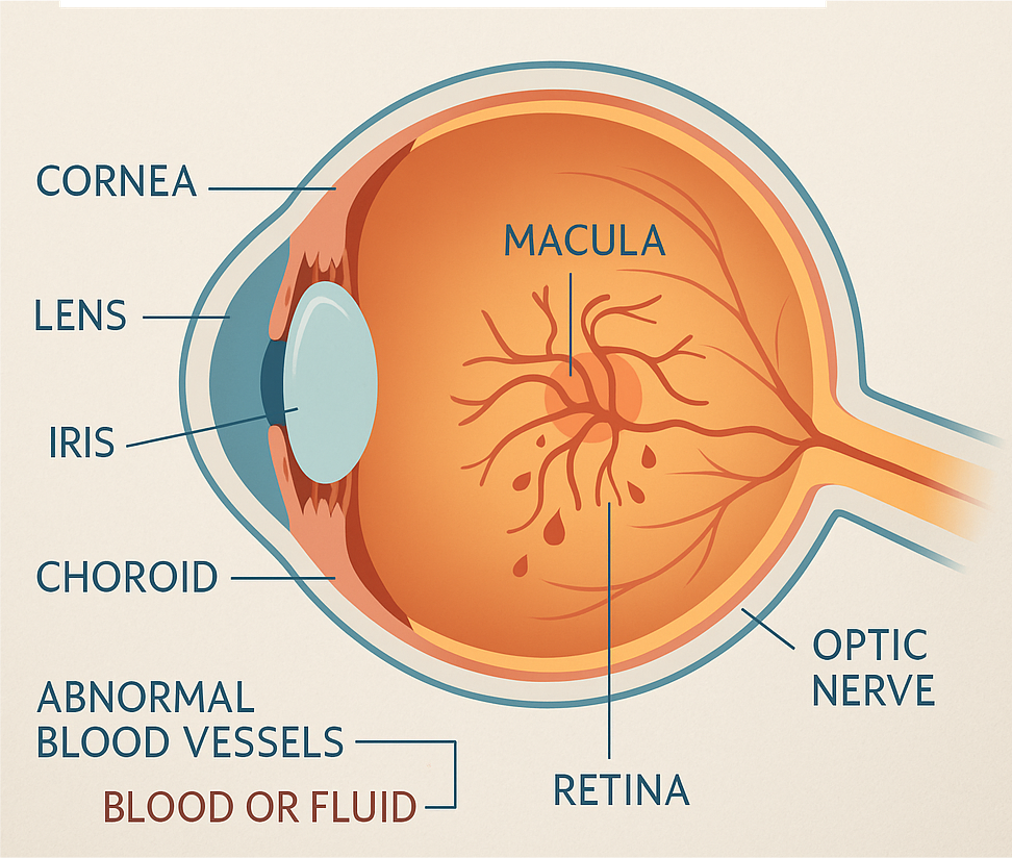
SCG3 has been shown to selectively regulate pathological—but not normal—angiogenesis in multiple models, including:Wet AMD (laser-induced CNV)
Retinopathy of Prematurity (ROP) (OIR model)
Diabetic Retinopathy (DR)
VEGF-independent:
SCG3 does not bind VEGF receptors. Despite the cognate receptor not yet being identified, SCG3 activates angiogenesis through VEGF-independent mechanisms, making it an entirely new therapeutic axis.Validated efficacy:
Across several preclinical models of DR, wet AMD, and ROP, neutralizing SCG3 antibodies have replicated anti-VEGF levels of efficacy while demonstrating a superior safety profile.
Mission Statement
Our mission is to bring safe and precise anti-angiogenic medicines to patients of sight-threatening retinal diseases who currently have limited or no effective options.
What We Do
Angira Therapeutics is a Fannin-founded biotechnology company focused on developing first-in-class, non-VEGF therapeutics for retinal diseases driven by pathological angiogenesis. As a Fannin-founded company, we benefit from extensive operational support across translational research, preclinical development, regulatory strategy, and company formation.
Our Model
Angira Therapeutics operates within Fannin’s capital-efficient development framework, leveraging a shared management team of experienced entrepreneurs and scientists to advance our innovative therapeutic programs.
Fannin’s model emphasizes disciplined, milestone-driven development while prioritizing non-dilutive funding to de-risk technologies before significant capital investment. In alignment with this approach, Angira has secured SBIR Phase I funding from the National Eye Institute, supporting early development of our SCG3-targeted Raptamer™ therapeutics.